Introduction:
One is left with very few options in patients with steroid refractory Gut Graft versus Host Disease (GVHD) which occurs post allogenic stem cell transplant (AlloSCT) with most of them increasing the financial burden, especially in resource poor countries like India and many others. Many studies looking in to the pathogenesis of Gut GVHD have confirmed the hypothesis that patients with less diverse faecal microbiome have a greater chance of developing intestinal GVHD and are more prone to succumb to transplant-related complications. Looking at the mechanism of cure in various diseases of the gut, led us to the thinking that reversal of intestinal dysbiosis could improve Gut GvHD. We used FMT as a second line treatment in few of our patients and got encouraging results. Our findings indicate the safety and effectiveness of FMT in acute Gut GVHD.
Patient details:
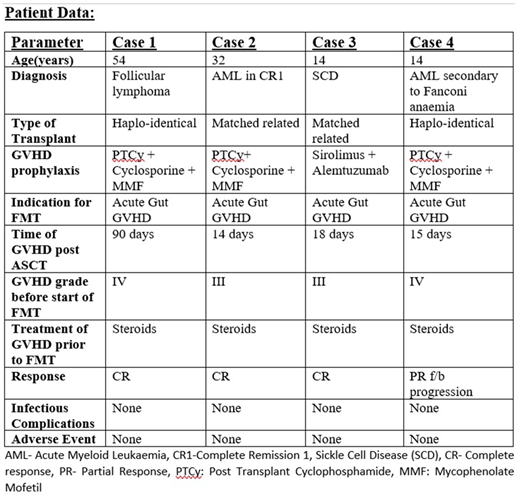
Please see image
Materials and methods:
Fecal material for FMT was taken from healthy donors who passed screening for transmissible disease. 100 gm of stool was prepared into a 200 ml solution and given once daily, serially for 4 days through naso-jejunal tube over 8 to 10 minutes. All patients received FMT as second line treatment after they were found non-responding or partially responding to steroids. All adverse events (AEs) that were first noted within 1 week of infusion of FMT were evaluated in terms of the safety. Response to FMT was evaluated at 14 days starting from the 1st FMT dose or at the time of maximum response whichever was first. Complete resolution of loose motions and gastrointestinal (GI) symptoms was considered as complete response (CR). Decrease in severity of GVHD by at least one stage was considered as partial response (PR).
Results:
All four patients include were treated between 2018 to 2020. All of them were diagnosed with GI acute GVHD (Gut ± upper GI) on the basis of clinical, scopy (colonoscopy ± endoscopy) findings and histopathology. Case 2 and Case 4 had liver GVHD simultaneously with Gut GVHD. The median time for initiation of FMT was at 52.5(14-90) days from the day of AlloSCT. Three patients (Case 2,3,4) had GVHD immediately post AlloSCT, while Case 1 had GVHD at day 90. All patients had stool microscopy, culture and a stool BIOFIRE test sent to rule out viral, bacterial and parasitic infections. Clostridium difficile infection was not observed in any of the patients. Three patients (Case 1,2,3) received single course (4 days serially) while case 4 required 2 courses 14 days apart. Case one had sigmoid colon involvement and we also gave him per rectal FMT via a flatus tube which was placed in his sigmoid colon. The procedure was well tolerated by all patients. No side effects related to FMT were suspected or confirmed in any patients. Three patients (Case 1,2,3) achieved CR with complete resolution of loose stools and GI symptoms. The median time to response was 4 days (3-5 days). We were able to reduce the dose and taper off steroids in all four patients. Case 4 showed partial response but again had aggravation of GVHD after each course of FMT. At last follow up, two (case 2 and 3) out of four patients were alive. Case 1 expired 2 months after resolution of GVHD due to anginal attack. Case 3 had a secondary graft failure but is still transfusion independent. Case 4 expired due to sever uncontrolled acute GI GVHD (colonic + upper GI).
Conclusion:
In summary, FMT can be considered as a potential, relatively safe and well tolerated option for treatment of acute Gut GVHD in AlloSCT patients. Further clinical trials with large number of patients are required to explore FMT as one of the effective options for treatment of acute GVHD. Despite the small number of patients, previous findings and our data corroborate that interventions to maintain intestinal diversity can improve outcomes of AlloSCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.